Contact Us
- Solutions
- Resources
- About
- Contact Us
close
Optional callout banner for highlighted news or events
Learn More
The journey to developing and manufacturing small-volume parenteral (SVP) drugs is undergoing rapid change and innovation. As breakthroughs in biologics, personalized medicine, and global health issues increase the demand for SVPs, there is also a need for stringent regulatory compliance and state-of-the-art technologies that guarantee sterility and high-quality production.
Navigating these complexities requires understanding key market drivers that shape the SVP landscape, with a strong focus on risk mitigation. One of the most significant is the need for precision dosing and rapid onset of action, both critical for effective treatment. These demand attention during manufacturing to ensure each dose’s accuracy.
Additionally, advances in drug delivery systems, such as prefilled syringes, autoinjectors, and other medical devices, have improved SVP administration and patient convenience. However, these innovations bring new risks that must be managed through testing and quality control.
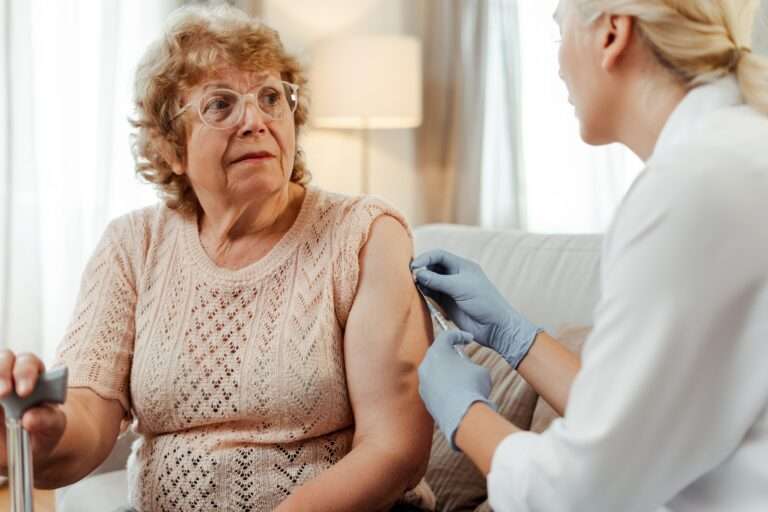
Furthermore, the increasing elderly population, often requiring in-home treatment, needs user-friendly, safe, and accurate devices. The rise in chronic diseases such as diabetes, cancer, Crohn's disease, and arthritis further emphasizes the importance of continuous innovation in SVPs. Managing the risks associated with new formulations and delivery methods is essential to ensure patient safety and treatment efficacy.
Despite the promising advancements and growing demand, the development and manufacturing of SVPs is challenging. SVPs have a unique set of requirements:
In addition, the manufacture of SVP drugs must adhere to strict regulatory guidelines, including:

Selecting a CDMO to manufacture your SVP drugs is a critical decision. It involves evaluating their expertise, regulatory compliance, manufacturing capabilities, project management skills, cost structure, and support services to confirm they align with your needs and regulatory requirements.
When choosing a CDMO partner, consider the following key factors:
Experience and Expertise: Look for a CDMO with a proven track record in SVP development and manufacturing. Their team should possess in-depth knowledge of aseptic processing to ensure the sterility and safety of your drug. Expertise in formulation development, analytical chemistry, and regulatory affairs is also essential for navigating the complexities of SVP projects. Select a CDMO that prioritizes patient safety by adhering to Good Manufacturing Practices (GMP) and holding certifications from major regulatory agencies like the FDA and EMA.
Open Communication and Transparency: Effective communication is vital throughout the development and manufacturing process. Your CDMO should provide dedicated project teams who keep you informed with clear timelines and consistent progress updates. Look for a partner who fosters transparency by offering detailed breakdowns of costs associated with development, manufacturing, testing, and other services. This allows you to manage your budget effectively.
Flexible Approach for Project Success: The regulatory landscape and project requirements can evolve during development. Choose a CDMO that demonstrates flexibility in accommodating changes to project scope, timelines, and regulations. Their responsiveness in addressing urgent requests and adapting to unforeseen challenges ensures a smooth project execution and ultimately, a successful outcome.
Pii: Your Partner in Safe and Efficient SVP Drug Development and Manufacturing
At Pharmaceutics International, Inc. (Pii), we support clinical development and commercial manufacturing for small- to medium-volume products and adapt to market demand changes. Whether transferring an existing product or developing one from scratch, clients are assigned a Pii formulation scientist as their project lead, ensuring continuity through to commercial manufacturing.

This scientist works with a dedicated team throughout the project lifecycle, providing easily accessible support that accelerates the entire process, from development to commercialization. Clear and transparent communication is key to our flexible approach, which allows for adjustments to project scope and timelines.
In addition, patient safety and regulatory compliance is one of our top priorities. Our facilities are equipped with cleanroom environments and advanced sterilization techniques. We comply with Good Manufacturing Practices (GMP) and hold certifications from major regulatory agencies like the FDA and EMA. Additionally, robust quality assurance (QA) and quality control (QC) processes ensure all products meet stringent quality requirements.
The small volume parenteral (SVP) market is experiencing significant growth driven by factors such as personalized medicine and rising chronic diseases. While this surge presents exciting opportunities, it also necessitates robust risk mitigation strategies to ensure patient safety and product efficacy. By understanding the unique challenges of SVP development and manufacturing, companies can implement effective control measures throughout the process.
Partnering with a qualified CDMO like Pii with expertise in aseptic processing, regulatory compliance, and quality control can significantly reduce risks and help circumnavigate the complexities of bringing these medications to market.
Learn more about Pii’s parenteral drug solutions: https://www.pharm-int.com/formulation-process-development/parenteral-drug-development/

Sam Chia is the Senior Director of Operations MS&T at Pharmaceutics International, Inc. (Pii), with over 26 years of experience in aseptic manufacturing and sterile processing. He has held several leadership positions, including Director of Aseptic Operations and Plant Manager at ImprimisRx, a division of Harrow Pharmaceuticals. His career spans roles in production and operations optimization. Sam is fluent in both English and French, which complements his extensive international experience in pharmaceutical manufacturing and operational management across the United States and beyond.
Sam has been involved in several startup Operations in several big pharma companies including Ben Venue Laboratories a division of Boehringer Ingelheim and Cangene Biopharma formerly.
known as Chesapeake Biological Laboratories now Bora Pharmaceuticals. Sam is an associate Member of the Royal society of Chemist England and Holds a BS degree in chemistry with a biochemistry minor.
Like what you read? Share with your network: